WMIF MAIN SITE
2026 Event Site
The day opened with a conversation between Arpa Garay, Moderna’s Chief Commercial Officer, Geoff Meacham, PhD, Managing Director, Global Research for BofA Securities, and Paula Ness Speers, Co-Founder and Senior Advisor of Health Advances. Garay is excited about a possible future combination flu/COVID annual shot, as well as customizing flu vaccines by country and strain. She noted the future potential for an RSV vaccine in a pre-filled syringe with a similar safety profile and, ultimately, a combination flu/COVID/RSV vaccine for older adults.
In oncology, Moderna is focusing on a neoantigen-based therapy that could help individualize treatment of non-small cell lung cancer tumors. Garay said working alongside Merck and their immunotherapy treatment, Keytruda, could be game-changing.
Watch Fireside Chat with Arpa Garay
Antibodies are an underlying factor for new approaches in cancer treatments, said a panel of executives who spoke with moderator Daniel Haber, MD, PhD, Director of the Mass General Cancer Center.
Common light chain, an approach that simplifies how proteins bind to multiple things, might help create a personalized method to treat difficult cancers such as lung, head, and neck, said Bill Lundberg, MD, CEO, Merus.
Another strategy is the development of IGM antibodies, which has 10 binding domains versus the two found in IgG antibodies. “With this, we believe it can bind to more difficult targets,” said Fred Schwarzer, CEO, IGM Biosciences. “It’s like holding onto something with two fingers versus two hands.”

Congratulations to Li Chai, MD, of Brigham and Women’s Hospital, and Clotilde Lagier-Tourenne, MD, PhD, of Massachusetts General Hospital, the winners of the First Look Award!
The research in the Chai lab addresses transcription factors (TFs), gene regulation, and their therapeutic applications, particularly in cancer. They have studied the oncogenic TF protein SALL4 for 20 years and found that it plays a causative role in cancer development. These findings demonstrate the significant potential of SALL4 as a cancer target, leading to a promising new approach for cancer therapy.
Lagier-Tourenne’s presentation focused on alterations in RNA metabolism that have become a key focus in understanding neurodegenerative diseases such as ALS, Frontotemporal Dementia (FTD), and Alzheimer’s Disease. Through comprehensive studies, TDP-43 has been identified as a crucial RNA-binding protein that regulates the expression and splicing of important neuronal growth-associated factor, stathmin-2. The restoration of stathmin-2 alone has shown remarkable potential in maintaining the connection between motor neurons and muscles, making it an attractive therapeutic strategy for patients. Both presentations addressed an unmet need with strong scientific and collaboration potential and embodied the innovative, entrepreneurial, and visionary spirit that the World Medical Innovation Forum was established to recognize. Congratulations to the First Look winners and panelists on an excellent series of presentations and many thanks to the expert Bank of America judging panel.
Watch Li Chai, MD’s First Look Presentation
Watch Clotilde Lagier-Tourenne, MD, PhD’s First Look Presentation
Given the recent market downturns, trends in medical research and biotech funding are top of mind. Greg Butz and Sumit Mukherjee, Managing Directors at BofA Securities, were joined by Yvonne Hao, Secretary of Economic Development for the Commonwealth of Massachusetts, for a lively discussion on the future of healthcare investment and how to continue to drive innovation from the lab to the clinic.
In the past, biotech venture capitalists saw “crazy valuations and very little (healthcare) improvements, when people thought money was falling from the sky,” but according to Craig Gordon, MD, CEO, GordonMD, all that has changed with today’s greater pricing pressures and regulatory pressures. Companies that approach VCs for funding now will need the right management team, the right shareholder base, and a long-term horizon along with the right valuation. “We need to get back to basics,” he said.
Such boom-and-bust cycles are normal, said panelist Roger Kitterman, Senior Vice President, Venture, Business Development and Licensing at Mass General Brigham. “Cycles tend to be long, and they’re hard to predict. But we need to build companies that can handle that.”
Hao, who has an extensive background in venture and start-ups, shared her enthusiasm for investment in innovation and companies and investors working together long-term. “It’s not just the cash,” she said, “the marriage is important.”
Further exploring the investment landscape of healthcare innovation, Greg Butz Managing Director, Co-Head, North American Healthcare Investment Banking, Head of Life Sciences Investment Banking, BofA Securities, and Adrian Mee Managing Director, Head of Global Healthcare Investment Banking, BofA Securities hosted the M&A and Business Development Roundtable.
Hear more from Secretary Yvonne Hao on Massachusetts innovation
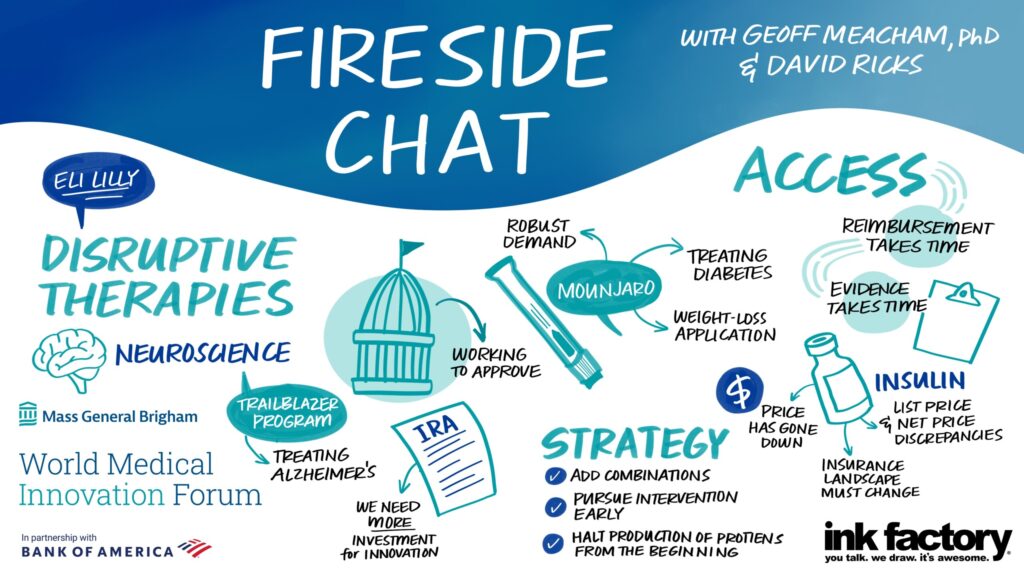
Addressing costs for its new blockbuster obesity drug Mounjaro, and longtime diabetes insulins like Humalog, Eli Lilly and Company CEO David Ricks talked about how to manage costs and supply for both.
“We launched Mounjaro last June and we will break every record in our company for adoption,” he said. “I’ve never launched something we ran out of so quickly.”
While the medication has been prescribed off-label for obesity, Ricks said that its use for type 2 diabetes to delay or halt the need for insulin use might be a way to “try to hit it with the best tools first and get in front of that. We hope to knock the disease backward.”
Studies are ongoing to see how Mounjaro helps with sleep apnea and cardiovascular issues. “Our aim is bold here, and we know obesity is the key to disease progression way beyond diabetes and metabolic conditions. Perhaps we can improve the health of many people through this class of medications,” he said.
Ricks also cited Lilly’s efforts to reduce insulin costs to $35 a vial. “We did this as leadership to get out of the conversation about drug price or insurance design and I am waiting for insurance design to change,” he said, adding that the country needs to get out of a system where patients with high-deductible insurance plans shoulder the full out-of-pocket cost of medications before meeting their deductibles.
Watch Fireside Chat with David Ricks
The Advanced Research Project Agency for Health (ARPA-H), is a new federal endeavor that tackles high-payoff research — a new agency taking a fresh approach to health in the U.S.
“This is a moonshot that the rest of the funding ecosystem can’t take on,” said ARPA-H Director Renee Wegrzyn, PhD, who became the agency’s first leader in fall 2022.
Project managers, or “demonstrated doers” as Wegrzyn described them, are hired for three-year terms to develop solutions for vexing health problems, using a common framework addressing accessibility, cost, and user experience. ARPA-H has an initial Congressional appropriation of $2.5 billion.
Wegrzyn said the agency’s first project, known as Novel Innovations for Tissue Regeneration in Osteoarthritis, or NITRO, seeks to regenerate bone tissue using a person’s own cells, potentially reducing the need for traditional joint implants.
ARPA-H will have three “hub and spoke” locations in the U.S. that “represent all Americans,” said Wegrzyn. “We need to make sure we can transition programs outside of ARPA-H.” The agency is currently in a competitive process for selecting two sites: a Customer Experience Hub that can recruit for clinical trials and scale manufacturing, and an Investor Catalyst Hub to convene venture investment and funding.
“We are very aware that the gap in innovation and how things work in the real world is vast,” she said. “We want to bridge that gap.”
Watch Fireside Chat with Renee Wegrzyn, PhD
In a lively discussion with Robert Califf, MD, Commissioner of the U.S. Food and Drug Administration, co-moderated by Tazeen Ahmad, Managing Director of Global Research, BofA Securities, and Lindsey Baden MD, Vice President of Clinical Research, Brigham and Women’s Hospital, Califf spoke about the challenges and opportunities in today’s regulatory environment.
Baden asked if the FDA had the teeth to reassess drugs that had been approved under what he called imperfect data.
“We’ve got the teeth, but we don’t have the system,” said Califf, who led the COVID vaccine trials for Moderna. “Why do I have to call Israel or the UK with the next dose of the vaccine? Because they are using their EHRs (for data) and they answer questions quickly.” When U.S. agencies and other healthcare stakeholders such as researchers, regulators, and payers are fully aligned, as they were when developing the COVID vaccine, “it’s amazing what can be done in America where miraculous things can happen,” he said.
Watch Fireside Chat with Robert Califf, MD
