WMIF MAIN SITE
2025 Event Site
Director, Familial Dementia Neuroimaging Lab and Director, Multicultural Alzheimer’s Prevention Program, MGH; Paul B. and Sandra M. Edgerley MGH Research Scholar; Associate Professor, HMS
For the past two decades, Dr. Quiroz and the Mass General Familial Dementia Neuroimaging Lab have followed an extended family of 6,000 individuals with a known Alzheimer’s disease (AD) genetic mutation, PSEN1 E280A. The work with this family has revealed key details about the genetics and early brain change associated with AD. This study was presented at the 2023 World Medical Innovation Forum.
In 2014, Quiroz launched the COLBOS (Colombia-Boston) project, an international collaborative longitudinal biomarker study to identify the earliest in vivo pathological and functional abnormalities associated with autosomal dominant AD. Dr. Quiroz and her team have identified a few COLBOS study participants who, despite carrying the PSEN1 mutation, remained cognitively unimpaired until a relatively old age compared with others with the same mutation, who exhibit cognitive impairment at a median age of 44 years prior to developing dementia at 49 years. These rare occurrences yield insights into potential protective factors against the neuropathogenesis of AD and possible avenues for therapeutic targets.
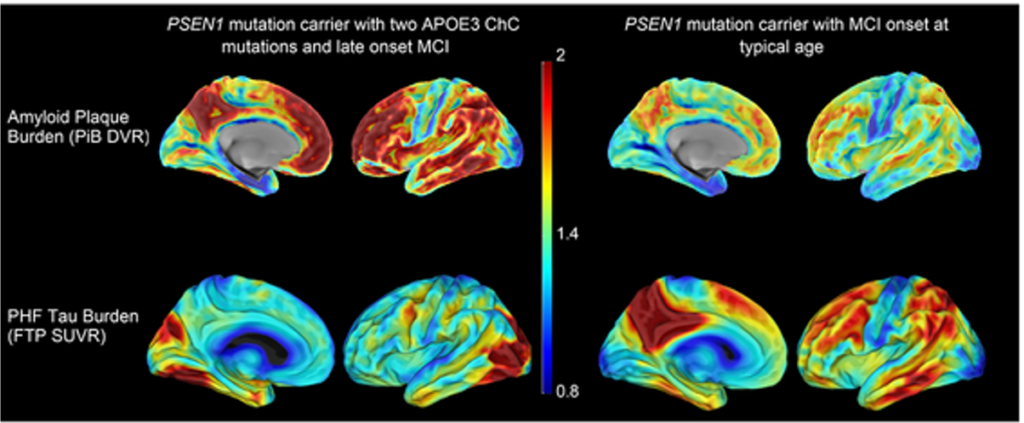
One of these late-onset COLBOS study participants was a woman who began showing signs of AD thirty years after the expected age of clinical onset. Clinical and PET imaging studies of this individual showed an extremely high amyloid level but a tau burden and neurodegeneration lower than expected for her age. These findings suggest a disconnection of amyloid pathology from tau pathology, neurodegeneration and cognitive impairment (Arboleda-Velasquez… Quiroz, Nature Medicine, 2019) (Fig. 1). Genetic analyses revealed a homozygous rare variant of APOE3 (R136S substitution, known as the Christchurch variant, APOECh), which plays a role in binding to lipoprotein receptors and heparin sulfate proteoglycans (HSPG). Experimental studies showed that this mutation impairs heparin binding to ApoE. Our findings suggest that antibodies or small molecules binding to the R136S-containing APOE region or otherwise modulating APOEHSPG interactions could reproduce this potentially protective effect of APOE3Ch, representing a promising novel target for AD therapies.
Case studies like this APOE3Ch case have a high sensitivity for novelty: they may reveal critical, previously unrecognized pathways and mechanisms of cognitive resilience and resistance to AD and ultimately lead to novel, patient-inspired therapies for AD and other neurodegenerative diseases.
Learn more about the 2023 World Medical Innovation Forum First Look presentations.