WMIF MAIN SITE
2025 Event Site
Associate Bioengineer, BWH; Associate Professor of Medicine, HMS
The Artzi lab is designing biomaterials that enhance precise drug delivery to enhance therapeutic outcomes in a range of diseases. Immune modulatory drugs can help regulate the immune system and generate a ‘living,’ long-lasting response. To realize the full potential of immunotherapies, there is a need for non-viral delivery systems targeted delivery and enhanced uptake to specific cells. Yet, when biological barriers to delivery limit drug accumulation in particular organs, such as in the brain, the combination of nonviral nanomaterials with macroscopic materials that can be injected in a particular site may expand the therapeutic window. We engineered an injectable dendrimer:dextran-based adhesive hydrogel that gels rapidly upon application and adheres to a tissue target. This hydrogel can be loaded with a therapeutic drug to enable localized and sustained drug delivery, bypassing the barriers associated with systemic administration.
Potential applications of this technology include autoimmune diseases and cancer, including glioblastoma (GBM)—an aggressive form of brain cancer with a median overall survival rate of 15 months. Treatment for GBM involves surgical resection followed by chemotherapy and radiation therapy. Yet, tumor recurrence is inevitable as it is impossible to eliminate all tumor cells with current strategies. The development of more efficacious therapies is hindered by the low permeability of the blood brain barrier (BBB) to systemic therapies, GBM’s diffuse and infiltrative nature, and the immune quiescence in the brain. Local delivery enables the use of drugs irrespective of their ability to cross the BBB and enhances their therapeutic efficacy and tolerability.
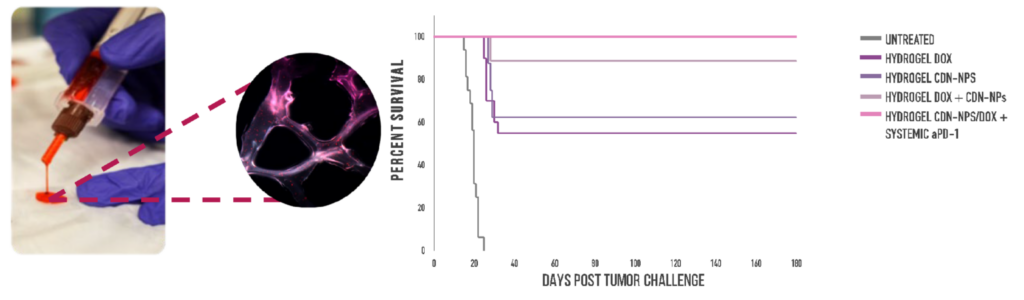
We leveraged our injectable, adhesive hydrogel to mediate controlled local delivery of chemoimmunotherapy in the brain for GBM (Fig. 1). We demonstrated the use of this system for local and sustained release of the immunogenic chemotherapy doxorubicin in combination with a proprietary nanoparticle formulation of cyclic dinucleotides, which are stimulator of interferon genes (STING) agonists that promote an immunostimulatory tumor microenvironment. In vivo experiments in immunocompetent clonotypic murine models of GBM demonstrated that local hydrogel-mediated delivery of this chemoimmunotherapy eliminated tumors in 90% of the mice and generated anti-tumor immune memory (Fig. 1). In contrast, intratumoral delivery of the same chemoimmunotherapy resulted in rapid drug clearance and did not elicit immune memory, demonstrating the value of sustained immunotherapy delivery. This platform technology can be exploited to deliver a range of combination therapies, overcoming delivery barriers and enhancing therapeutic outcomes while minimizing side effects in GBM and other oncology and autoimmune diseases.
Learn more about the 2023 World Medical Innovation Forum First Look presentations.